Cancer
Cancer is a leading cause of premature death in every country in the world (Cancer Atlas) and it is estimated that one in three people will be affected by the disease in the UK.
Cancer researchers at Brunel University London aim to increase our understanding on different hallmarks of cancer development and progression, in particular within CIRTM, we focus on cancer immunology and cancer metastasis.
We investigate cellular and molecular factors in childhood cancers like neuroblastoma, and adult cancers such as melanoma, gastrointestinal, breast and prostate cancers. The primary research themes at CIRTM include oncogenic transcription factors, immunity against cancers, cell-signalling, transcriptional deregulation, and targets for clinical intervention.
To investigate these key mechanisms, we use a range of cutting-edge techniques and models, including advanced microscopy, transcriptomics, in vivo imaging (MRI/PET/optical), transgenic preclinical animal models, and non-animal models, such as microfluidics and human organ-on-a-chip in vitro models. These innovative approaches aim to provide deeper insights into cancer biology and develop more effective, diagnostic biomarkers and targeted therapies for patients.
Our ultimate goal is to establish approaches to improve treatment and management of cancer in clinics.
Neuroblastoma
It is the most common and aggressive extracranial solid tumours occurring in childhood. It remains a major cause of cancer-related deaths in infancy. Around 100 children in the UK are diagnosed with neuroblastoma each year and half will be high-risk. This is equal to six out of 100 of all childhood cancers diagnoses. Despite therapeutic strategies based on chemotherapy, radiotherapy, surgery, GD2-targeted immunotherapy, stem cell transplant and treatment with 13-cis-retinoic acid, high-risk neuroblastoma outcome remains poor, with a 5-year event-free survival <40%. Tumours show initial response to therapeutic interventions but typically relapse into an incurable form of the disease. Moreover, several drugs cause severe side effects, including cognitive impairment and retarded growth. Thus, to reduce drug toxicity and to improve the outcome and the lifestyle of the patients affected by neuroblastoma, additional therapeutic options are required.
Professor Arturo Sala’s Lab: We are developing new anti-cancer immunoconjugates for neuroblastoma targeting the MYCN oncogene. We are also repurposing selective serotonin reuptake inhibitors, such as Prozac, to combat neuroinflammation and reactivate the anticancer immune response.
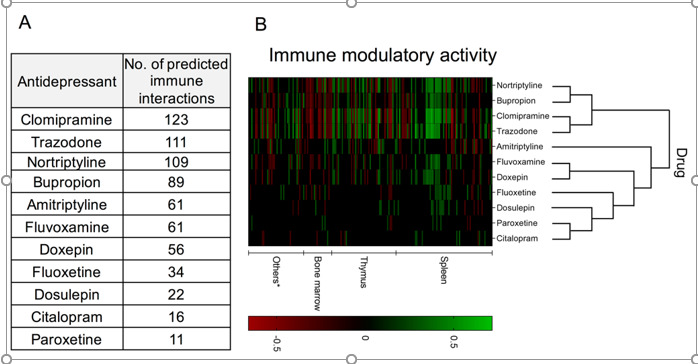
Comparative immunomodulatory activity of 11 antidepressants generated by data-mining the computational predictions generated by Kidd et al. (2016) for 1,309 drugs and 221 immune cell states. This predictive approach is based on the assessment of the similarity between transcriptional signatures of immunological state changes (ImmGen database) and drugs tested in vitro (Connectivity Map database). This assessment was used to compute an immunemod score based on the overlap of the top- and bottom-ranked genes in each profile. Considering an immunological state change from A to B, a positive immunemod score indicates that the drug can bias the immune cell toward state B, whereas a negative score indicates that the drug may shifts the cell toward state A. (A) Table indicating the total number of predicted immune interactions; (B) Hierarchical clustering and heat map indicating the immunemod score for each predicted immune interaction in different organs and inter-drug similarity. Columns and rows indicate, respectively, specific immune interactions and drugs.
Breast and Prostate cancer metastasis
Breast and prostate cancers are the first and the fourth most diagnosed solid cancers worldwide, respectively. More than half of the new cancer cases in the UK are breast and prostate. Primarily, ~90% of cancer-related deaths for breast and prostate cancers are due to cancer spreading to a different part of the body forming a secondary tumour called metastasis. Breast and prostate cancer metastasis can form in secondary organ as lungs, bone and brain, as a result of cancer cells leaving the primary tumour and invading the surrounding to reach the bloodstream. Here, cancer cells can interact and adhere to the endothelial cells forming the blood vessels of secondary organs followed by transmigration and growth of cancer cells in metastasis. Despite clinical treatments including surgery, radiotherapy, chemotherapy, hormone therapy, metastasis is a big challenge in healthcare due to ineffective clinical treatments. Metastasis steps underline molecular mechanisms are not fully understood and the clinical trials failure show a need for further research to identify metastasis formation targets.
Dr Camilla Cerutti’s Lab: We are investigating the molecular mechanisms and signalling of early steps of metastasis, such as cancer adhesion and migration using microfluidic 2D and 3D vasculature-on-a-chip in vitro model to mimic in vivo features like hemodynamic shear stress.
Development of vasculature-on-a-chip for cancer metastasis studies
CIRTM Organ on chip research is formed by an international and multi-interdisciplinary group of experts from Life Sciences and Engineering developing microfluidic devices to mimic human tissues in order to replicate organ-like functions. The research and development of the vasculature-on-a-chip is part of the organ-on-a-chip (OOC) group’s at Brunel University London working on breast, vagina, ovary, and placenta-on-a-chip. Cerutti`s lab aims to study early steps of metastasis within the vasculature-OoC to develop a human 3D representative system, allowing a better understanding of metastasis and to identify targets for better treatments.
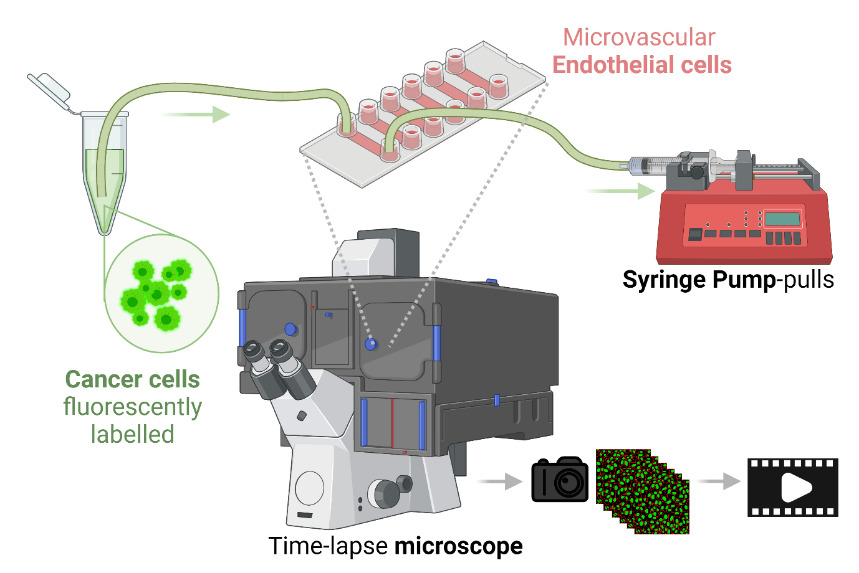
Microfluidic workflow to study early steps of cancer metastasis with high-content imaging (Cerutti et al. 2016, 2017, 2021, 2022, 2023, 2024). Image created with Biorender.com.
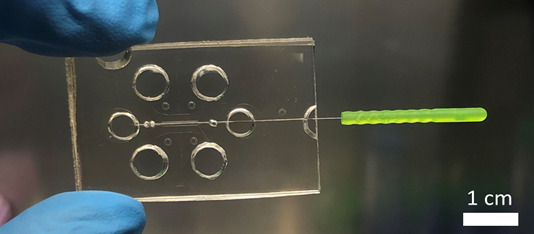
Picture of a 3D organ-on-a-chip device used in Dr Cerutti’s lab.
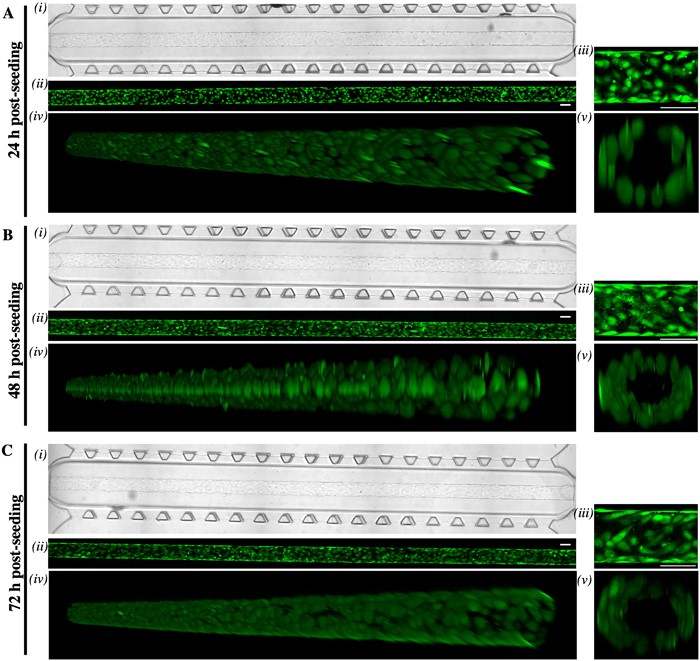
Images of endothelial cells growing in the 3D organ-on-a-chip device used in Dr Cerutti’s lab.
Cancer Immunology
Cancer immunology is the study of how the immune system interacts with cancer. Normally, the immune system protects our bodies by identifying and destroying harmful invaders like viruses and bacteria. However, cancer cells can sometimes trick the immune system or hide from it, allowing the cancer to grow unchecked. Immunotherapy, a promising new approach, helps the immune system fight cancer more effectively. Treatments like cancer vaccines and immune checkpoint inhibitors work by boosting the immune system's ability to find and destroy cancer cells or by preventing cancer cells from hiding. Although immunotherapy is increasingly used in clinics, not all patients benefit from treatment, and some may experience autoimmune side effects that require careful monitoring. To improve these treatments, researchers are working to better understand how cancer interacts with the immune system and to develop biomarkers and diagnostic tools for treatment monitoring and matching the right treatment to the right patient.
Dr Doreen Lau’s Lab: We are investigating the biological mechanisms that govern cancer immunity, how patients respond to immunotherapy, and why some treatments fail. To do this, we employ advanced techniques to profile cancer and immune cells at the genetic, protein and metabolic levels in both patients and preclinical models. Additionally, we use chemical biology and engineering approaches to develop new tools for tracking immune cells and imaging the entire body. These efforts aim to gain deeper insights into how immune cells interact with cancer cells, whether at the level of individual cells, within specific tissues, or across the entire body.
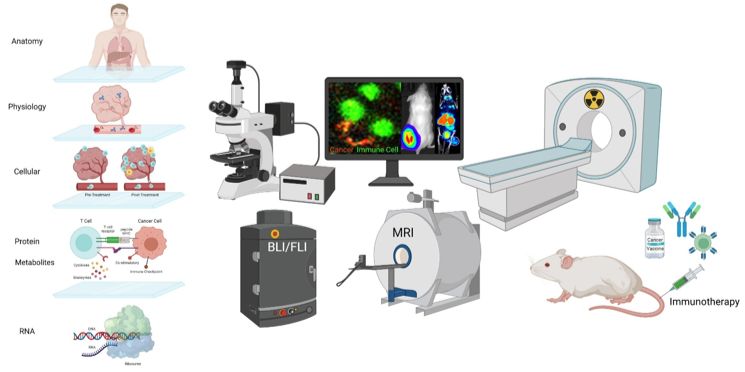
Understanding cancer immunity, immunotherapy response and treatment resistance from single-cell to whole-body level (Lau et al. 2020, 2021, 2022, 2023). Image created with Biorender.com.
Academics working in Cancer research theme: